Abstract
Background: Circulating tumor DNA (ctDNA) dynamics has been proven to be a reliable outcome prediction biomarker in a plenty of solid tumors. However, its clinical utility in response assessment and prognosis prediction for diffuse large B-cell lymphoma (DLBCL) has not been well established. This study was aimed to explore the association between ctDNA dynamics and clinical benefits in a cohort of DLBCL patients.
Methods: A total of 37 newly diagnosed DLBCL patients treated with first-line therapy were enrolled retrospectively in this study. Tumor biopsy tissues were collected at diagnosis (n = 17) and peripheral blood samples were obtained before treatment (n = 31) as well as after receiving the initial two treatment cycles (C2, n = 37). Tumor genomic DNA and cell-free DNA (cfDNA) were extracted from tumor tissues and plasma samples, respectively. Targeted next generation sequencing of 188 lymphoma associated genes (Onco-LymScan, Genetron Health) was performed in all of the tumor samples to identify disease-associated mutations. Genomic DNA extracted from the paired peripheral blood mononuclear cells were also sequenced and analyzed to filter out germline variants for each patient.
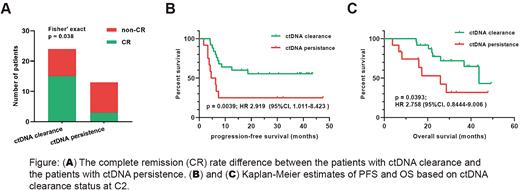
Results: All of the 37 patients were identified at least one somatic mutation from their pretreatment tumor and/or plasma samples. We sought whether these basal mutations could be traced in the C2 plasma of each patient. Mutations that were only detected in C2 plasma were considered uninformative and were removed from analysis. Accordingly, 12 patients were identified at least one basal mutation at C2 and 25 patients showed a rapid clearance of these basal mutations. Based on the ctDNA clearance status at C2, patients were classified into two groups: the ctDNA clearance group (n = 25) and the ctDNA persistence group (n = 12). There were no significant difference in serval clinical factors such as age, B symptoms, LDH (Lactate Dehydrogenase) level, stage and IPI (International prognostic Index) between the two groups. In response to the first-line treatment, patients with a ctDNA clearance showed a significantly higher complete remission rate (62.5% vs. 23.1%, p = 0.038; Figure A) than the patients with a persistence of ctDNA. With a median follow-up visit period of 26.0 mouths, patients with a ctDNA clearance at C2 presented a favorable progression-free survival (median PFS, unreached vs. 5.45 months; HR 2.919; p = 0.0039; Figure B) and overall survival (median OS, 43.6 vs. 25.87 months; HR 2.758; p = 0.0393; Figure C) compared with those without a ctDNA clearance. These results indicated that patients achieving an early ctDNA clearance during treatment were more likely to achieve a superior later clinical benefit.
Conclusions: Early ctDNA clearance during treatment could effectively serve as a predictive and prognostic biomarker for DLBCL patients receiving first-line therapy. Integrating ctDNA profiling into clinical disease management may help to identify patients at high-risk and contribute to individualized treatment.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal